Research Facilities has developed e-learning modules for mandatory annual safety trainings and refresher trainings.
Each research stream must take the following courses:
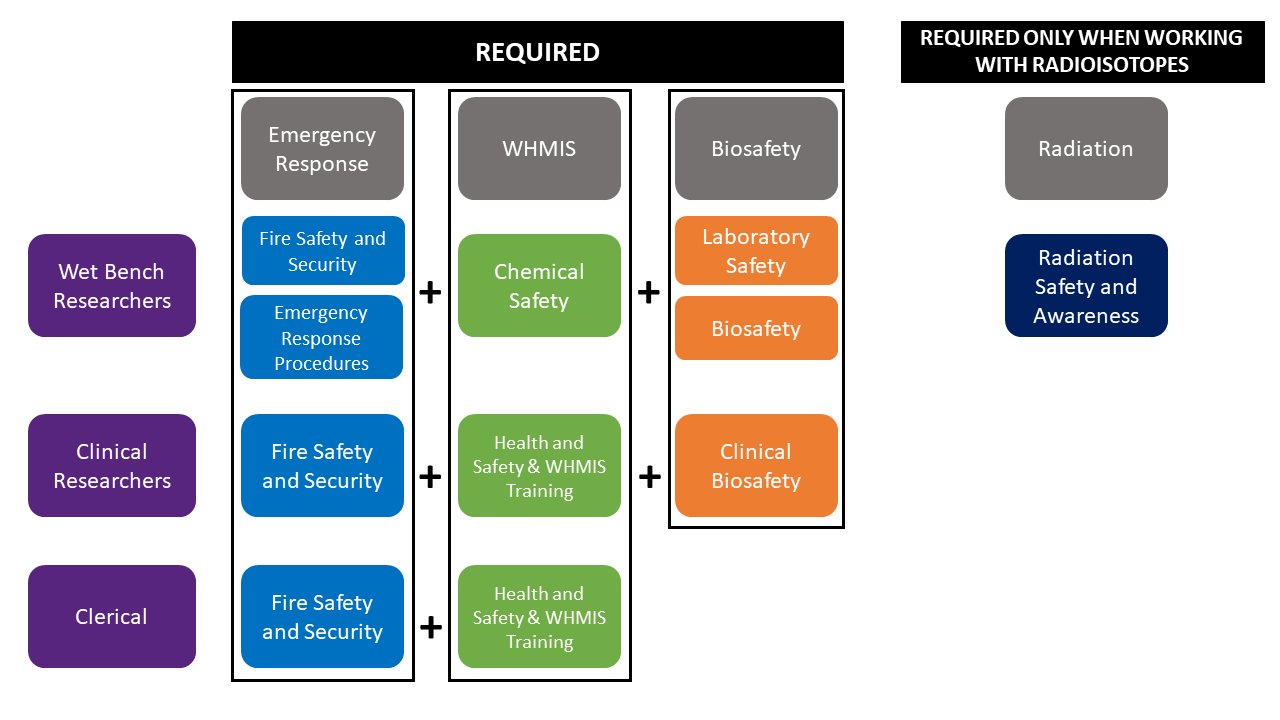
Wet Bench Researchers
Clinical Researchers
*Make sure you print screen the certificate/quiz results available at the end of the modules as proof you have completion of the training. This should then be stored in your group’s WHMIS binder. Please ensure that your name is on this document.
All new staff and students working in research areas must undertake safety training.
Clerical
Workers are required to complete WHMIS training available through the Unity Health Toronto Learning Centre for employees of the hospital (those staff members who have an employee number) or in our online learning section above for non-St. Michael’s workers.
Wet bench in-class training
Workers are required to attend a half-day biosafety training course within the first three months of starting work. This includes WHMIS regulations, risk group classification, risk assessments and the requirements for containment level 1 and 2 labs, basic laboratory biosafety, emergency spill procedures, and infection control practices. Please check our upcoming events for the next training session.
Clinical
Researcher co-ordinators II and III, that will be handling human specimens or undertaking lab work are required to attend a biosafety course designed to address clinical needs. Please contact the Biosafety Officer for more information.
iBEST Researchers
Please note that iBEST researchers do not have access to the staff Learning Centre. To access the hospital-required training modules, please use the Student Centre SRS portal.
Training sessions on blood borne pathogen, spill response and liquid handling are offered to all researchers.
Biohazardous and chemical liquid handling course
The purpose of the course is to:
To book a training session, please contact our Biosafety Officer.
Transport of dangerous goods
Staff involved in the shipping and receiving of hazardous goods are required to undergo Transport of Dangerous Goods (TDG) training and hold a valid certificate. Certificates are valid for three years under TDG regulations and two years under International Air Transport Association regulations.
Research Staff at St. Michael’s Hospital can obtain TDG training through N2 and CITI (Collaborative Institutional Training Initiative). N2 provides FREE access to the CITI-Canada program for N2 member organizations. If you are a member of N2 simply go to the CITI-Canada log-in page where you may access the program.
We don’t use the SSO option, so to register, users should not use the “Log in through my Organization” option.
Account Creation: To create an account users should go to the CITI website and click on “Register”. The organization affiliation is “Unity Health Providence St. Joseph’s and St. Michael’s Healthcare (N2)”. Please ensure you use your Unity Health email for registration.
Future Log Ins: For future visits, they would click on the “Log In” option (not “Log in through my Organization”).
Accessing the Course: Once logged in, there should be an option to view courses. If TDG doesn’t show up as a course that is ready to begin, the user can add it to their profile by selecting “Add a Course” closer to the bottom of the page.
Usage: Access to the Course is open to any Unity staff member or physician.
Cryogenic training
All staff wishing to handle cryogenic materials (liquid Nitrogen, “dry ice” CO2) must complete an online cryogenic handling course (please see online modules) and attend a hands-on demonstration with Dario Bogojevic. Access to the cryogenic dispensing area on the loading dock will only be given to those members of staff who have received training.
Additional training
To request a training session, please contact our Biosafety Officer.